The allure of penny stocks lies in their potential to deliver massive gains in a short period. However, in exchange for that opportunity, most penny stocks carry tremendous risk. They can be extremely volatile and are susceptible to “pump and dump” schemes and fraud.
Unlike regular stocks, the financial condition of most penny stock companies can be extremely difficult to analyze, as the majority of such stocks are traded on over-the-counter (OTC) exchanges, which are typically less transparent and less regulated than the major exchanges. In fact, in the penny stock space, it’s often easier to spot warning signs and red flags than it is to identify a sound investment.
Although the potential reward may make it worthwhile, choosing the right penny stock is a daunting task. Nevertheless, we’ll do our best to identify short-term trade opportunities in this exciting space.
With this in mind, we’re starting a new series called “Penny Stock of the Day”. These ideas are geared for traders with a high risk appetite.
Our Penny Stock of the Day is chosen by screening for stocks under $5 and then applying technical analysis on the shortlisted set of penny stocks showing unusual volume. When making these trades, please make sure to pay vigilant attention to pricing moves and have a strict stop loss in place to avoid significant losses.
Penny Stock of the Day: Endo International PLC (NASDAQ: ENDP)
Today’s penny stock pick is the specialty pharmaceutical company, Endo International PLC (NASDAQ: ENDP).
Endo International plc manufactures and sells generic and branded pharmaceuticals in the United States and internationally.
Its Branded Pharmaceuticals segment provides branded prescription products, including XIAFLEX to treat adult patients with Dupuytren’s contracture; SUPPRELIN LA to treat central precocious puberty in children; NASCOBAL nasal spray to treat vitamin B12 deficiency; AVEED to treat hypogonadism; PERCOCET to treat moderate-to-moderately-severe pain; TESTOPEL an implantable pellet indicated for TRT in conditions associated with a deficiency or absence of endogenous testosterone; EDEX to treat erectile dysfunction; LIDODERM a topical patch product containing lidocaine for the relief of pain; and products for the pain management and urology.
The company’s Sterile Injectables segment manufactures VASOSTRICT, a vasopressin injection; ADRENALIN, a non-selective adrenergic agonist; and APLISOL, which is a sterile aqueous solution, as well as generic sterile injectable products, including ertapenem for injections and ephedrine sulfate injections.
Its Generic Pharmaceuticals segment offers solid oral extended-release, solid oral immediate-release, liquids, semi-solids, patches, powders, ophthalmic products, and sprays. The company’s International Pharmaceuticals segment offers specialty pharmaceutical products in various therapeutic areas comprising attention deficit hyperactivity disorder, pain, women’s health, oncology, and transplantation.
Website: www.endo.com
Latest 10-k report: https://sec.report/Document/0001593034-21-000012/
Potential Catalysts / Reasons for the Hype:
- Investors responding positively to a WJHL report that days before jury selection was set to start in the Baby Doe opioid trial, Endo Pharmaceuticals made a settlement offer to the counties involved in Northeast Tennessee. The plaintiffs in the Baby Doe trial are government entities and an infant born drug-dependent being called “Baby Doe.” Three local prosecutors had filed a lawsuit against Endo, Purdue Pharma, and Mallinckrodt. Purdue and Mallinckrodt had filed bankruptcy, which left Endo as the only corporate defendant.
- Speculation Reddit taking an interest in ENDP.
- Upcoming earnings and forward statements.
- Citi reiterating its buy rating on Endo shares and maintaining its $11 price target.
On analyzing the company’s stock charts, there seem to be multiple bullish indications…
Bullish Indications
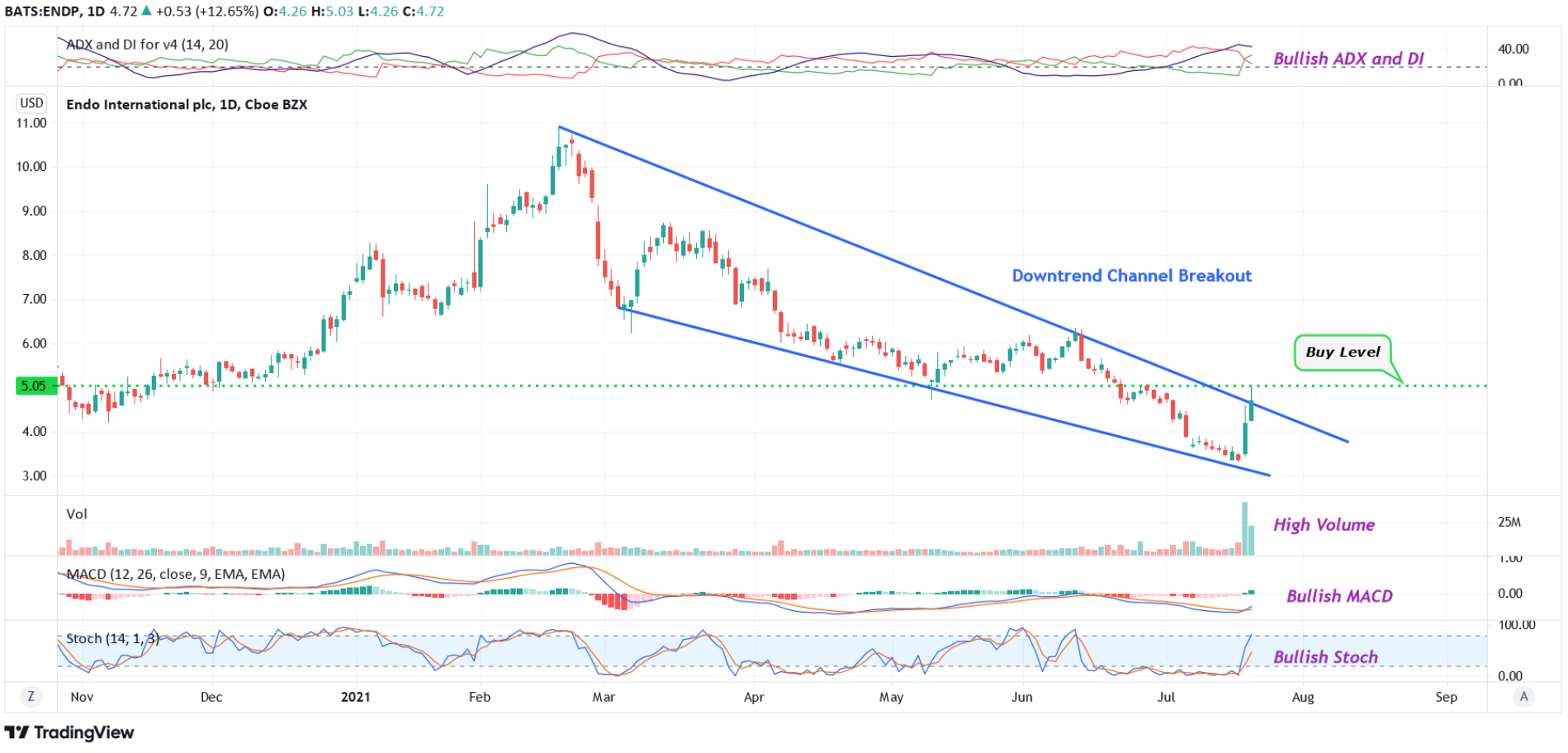
#1 Downtrend Channel Breakout: The daily chart shows that the stock was forming a downtrend channel during the past several months. This is marked in blue color. The stock had typically taken support at the bottom of the channel before bouncing back. The stock has currently broken out of the downtrend channel with very high volume, indicating possible bullishness.
#2 Bullish MACD: As seen from the daily chart, the MACD line (blue color) is currently above the signal line (orange color). This indicates a possible bullish bias.
#3 Bullish ADX and DI: The ADX indicator shows bullishness because the (+DI) line is greater than the (-DI) line, the ADX and (+DI) lines are above the (-DI) line, and the ADX line has currently moved up from below the (-DI) and (+DI) lines.
#4 Bullish Stoch: The daily chart shows that the stochastic is moving higher from oversold levels. The %K line is also above the %D line. All these indicate possible bullishness.
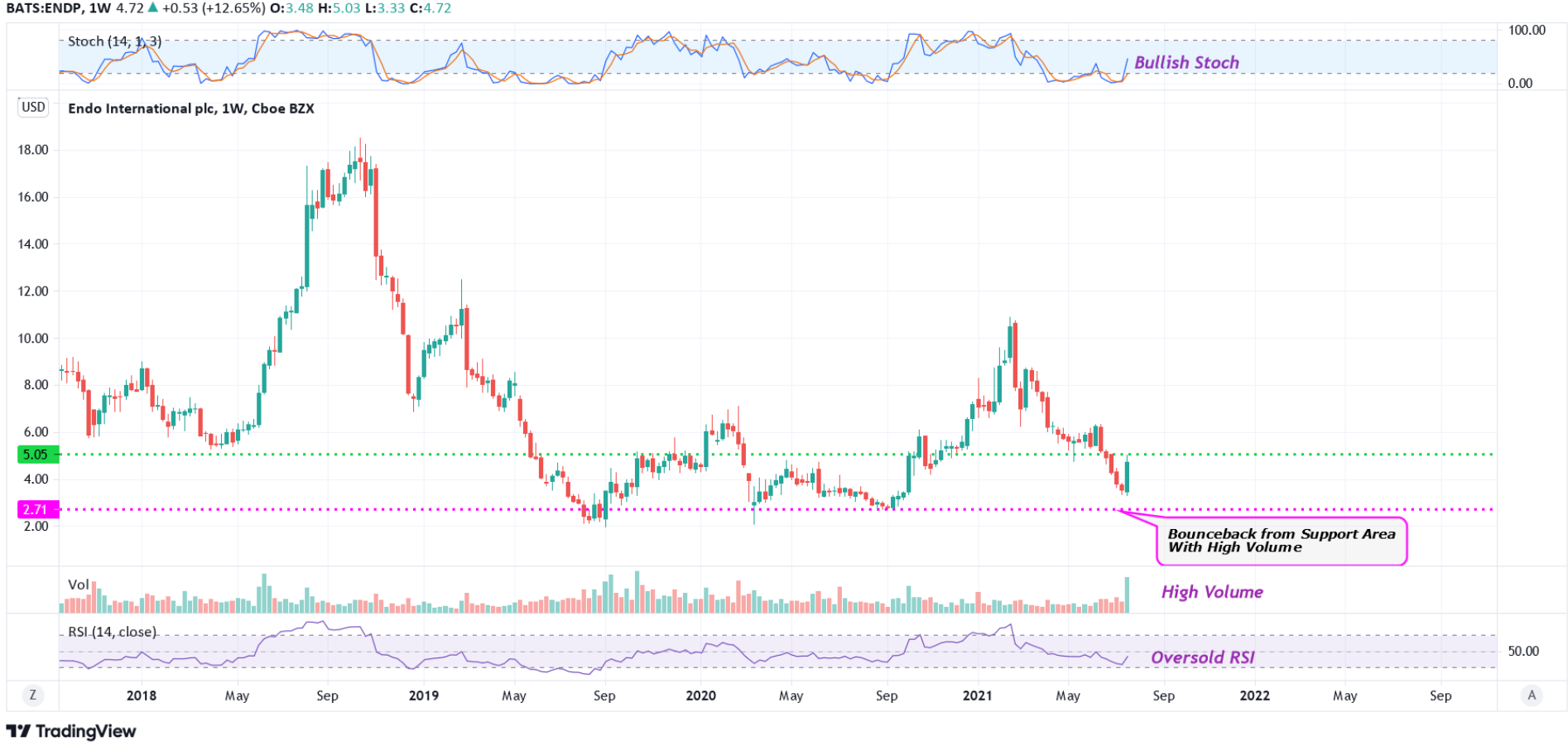
#5 Above Support Area: The weekly chart shows that the stock has broken out of a long-term support area with a very high volume. This has been marked as a pink color dotted line. This is a possible bullish indication.
#6 Bullish Stoch: The weekly chart also shows that the stochastic is moving higher from oversold levels. The %K line is also above the %D line. All these indicate possible bullishness.
#7 Oversold RSI: The RSI is currently moving higher from oversold levels in the weekly chart, indicating bullishness.
Recommended Trade (based on the charts)
Buy Levels: If you want to get in on this trade, the ideal buy level for ENDP is above the price of $5.05. This is marked as a green color dotted line in the chart.
Target Prices: Our target prices are $7.00 and $10.00.
Stop Loss: To limit risk, place a stop loss at $3.90. Note that the stop loss is on a closing basis.
Our target potential upside is 37% to 98%.
For a risk of $1.15, our first target reward is $1.95, and the second target reward is $4.95. This is a nearly 1:2 and 1:4 risk-reward trade.
In other words, this trade offers 2x to 4x more potential upside than downside.
Potential Risks / Red Flags:
- The company has an ongoing patent-litigation showdown with Eagle Pharmaceuticals over Vasostrict (vasopressin). Eagle is currently mulling an ‘at-risk’ launch. If done, it could cut into the profits of ENDP.
- The company has a history of discontinued operations. Since 2008, the company and its subsidiaries, including AMS (subsequently converted to Astora), was named as defendants in multiple lawsuits in various state and federal courts in the U.S., Canada, Australia, and other countries, alleging personal injury resulting from the use of transvaginal surgical mesh products designed to treat POP and SUI. As of December 31, 2020, the company has settled 71,000 filed and unfiled U.S. mesh claims.
- The cash flows from discontinued operating activities related to Astora included the impact of net losses of $63.5 million, $62.1 million, and $69.7 million for the years ended December 31, 2020, 2019, and 2018, respectively.
- The company was sued for alleged violations of antitrust law multiple times, of which some were settled.
- The company had to settle a putative class action entitled Public Employees’ Retirement System of Mississippi v. Endo International plc.
- As of February 18, 2021, the company is involved in approximately 20 cases filed by or on behalf of states; approximately 2,890 cases filed by counties, cities, Native American tribes, and/or other government-related persons or entities; approximately 300 cases filed by hospitals, health systems, unions, health and welfare funds or other third-party payers and approximately 185 cases filed by individuals.
- In addition to the lawsuits and administrative matters above, the Company and/or its subsidiaries have also received subpoenas, civil investigative demands (CIDs), and informal requests for information concerning the sale, marketing, and/or distribution of prescription opioid medications.
- The outcome of these lawsuits, CIDs, and subpoenas could have a material adverse effect on the company’s business, financial condition, results of operations, and cash flows.
- Even if Endo reaches a settlement with the Tennessee counties, the company’s legal battles related to its opioid drugs are far from over. According to New York Attorney General Letitia James, three major drug distributors had settled with the state of New York and two large counties in the state. However, a trial is still scheduled with Endo and two other drugmakers. Also, even if the company is able to do a settlement, it could lead to fear-of-missing-out FOMO buys and massive sell offs afterward.
- The company’s main revenue in 2020 was from the Sterile Injectables segment. However, there has been a decline in the company’s Branded Pharmaceuticals, Generic Pharmaceuticals, and International Pharmaceuticals segments. The company’s revenue has been slightly declining year over year. ENDP had generated total revenues of $2.90 billion, $2.91 billion, and $2.95 billion in 2020, 2019, and 2018, respectively.
- VASOSTRICT® accounted for 27%, 18%, and 15% of the company’s 2020, 2019, and 2018 net revenues, respectively. The increased revenues of VASOSTRICT® may not be sustained if Eagle Pharmaceuticals wins the patent litigation.
- The company has high debt that will take years to pay off. As of December 31, 2020, the company’s total debt is approximately $8.38 billion in aggregate principal amount.
- Endo is Under Investigation by the New York Department of Financial Services (DFS). The class action complaint against the Company for alleged violations of the Securities Exchange Act of 1934 between August 8, 2017, and June 10, 2020.
As you can see, today’s featured penny stock offers big upside potential… but it also comes with a number of risks and red flags. As always, when dealing with penny stocks, we advise caution before entering into such high-risk ventures. Remember to think before you trade… understand the risks… and if you decide to trade, stick to your stop-losses!
Happy Trading!
— Trades of the Day Research Team
Source: Trades of the Day